Ministry of Chemicals and Fertilizers
NIPER-A Technology Gift to Society for Rare Disease on Mahashivratri
Posted On:
01 MAR 2025 4:15PM by PIB Ahmedabad
NIPER Ahmedabad, an institute of National Importance under department of pharmaceuticals, Govt of India, has made a strategic alliance with Trident Lifeline Limited, a dynamic and rapidly expanding Small and Medium Enterprise (SME). This partnership, formalized through a technology transfer agreement, marks a significant milestone in bringing the critical cancer drug Vorinostat to Indian patients. The signing of the Memorandum of Understanding (MoU) by Prof. Shailendra Saraf, Director of NIPER-A, and Hardik Desai, Director of Trident Lifeline, on the auspicious occasion of Mahashivratri, symbolized the collaborative spirit and dedication behind this endeavour.
Currently, Vorinostat is not manufactured within India, forcing patients to rely on expensive imported versions, such as ZOLINZA, which command a price of approximately Rs. 14 lakhs. Recognizing the urgent need for a more affordable and accessible solution, a dedicated team at NIPER, under the astute leadership of Prof. Saraf and project investigator Dr. Ravi P Shah, embarked on a rigorous three-year research and development journey. The team, comprising Dr. Derajram Benival, Dr. Dinesh Kumar, Dr. Amol Dikundwar, Dr. Pinaki Sengupta, and Dr. Rajesh Nadiminti, along with enthusiastic NIPER students, meticulously worked on developing a generic version of Vorinostat. Their efforts encompassed the entire drug development process, from the synthesis of the Active Pharmaceutical Ingredient (API) to the precise formulation of the capsule.

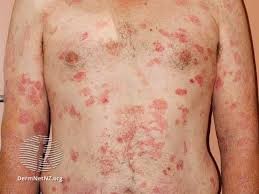
Vorinostat is one of the crucial medications for treating Cutaneous T-Cell Lymphoma (CTCL), a rare blood originated skin cancer. CTCL originates in T cells or T lymphocytes, vital components of the body's immune system. In CTCL, these cells undergo abnormal transformations, leading them to attack the skin. The disease manifests as scaly patches or bumps known as lesions, often mimicking eczema, which contributes to diagnostic challenges. The lack of readily available and affordable specialized treatment for CTCL in India has resulted in many patients being prescribed steroids, which offer compromised treatment with potential side effects.
Embracing the spirit of the Make-in-India initiative, Trident Lifeline Ltd. will scale up the production of Vorinostat and introduce it to the Indian market by leveraging NIPER Ahmedabad's expertise. This collaboration aims to revolutionize the treatment landscape for CTCL patients by providing access to a high-quality, domestically produced Vorinostat. Furthermore, demonstrating a profound commitment to social responsibility, Mr. Ashish Bafna, CFO of the Trident group, has promised to provide Vorinostat free of charge to economically disadvantaged patients. This compassionate initiative underscores the partnership's dedication to ensuring that life-saving medications reach those who need them most. In essence, this collaboration between NIPER Ahmedabad and Trident Lifeline exemplifies a powerful synergy between academic research and industrial innovation, driven by a shared vision of improving healthcare access and outcomes for the Indian population, particularly for those battling rare diseases.
AP/IJ/GP/JD
(Release ID: 2107276)