Ministry of Science & Technology
Giant antibody in humans acts like a brace against bacterial toxins
Posted On:
25 AUG 2025 5:51PM by PIB Delhi
A new property of the largest antibody so far identified in our body, changes our existing understanding of antibodies from chemical keys that fit into microbial locks to mechanical engineers, altering the physical properties of molecules to protect us
This could inspire new therapies by designing antibodies that mechanically stiffen dangerous proteins and disarming them.
Our immune system is armed with many types of antibodies, each with unique roles. Among them, IgM is the largest and one of the first antibodies our body produces when fighting infections.
A recent study from researchers at the S.N. Bose National Centre for Basic Sciences (SNBNCBS), an autonomous institute of the Department of Science and Technology (DST) reveals that IgM not only binds to pathogens but can also mechanically stabilize bacterial toxins, preventing them from harming our cells.
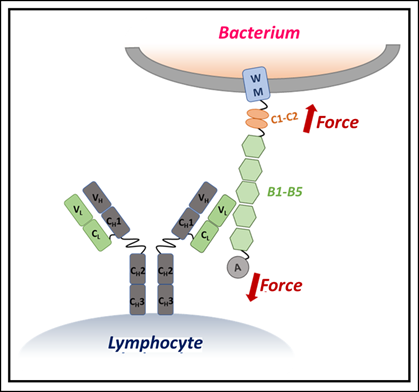
Fig: Protein L–antibody interaction- Protein L, secreted as a superantigen by Finegoldia magna, is composed of several domains, including membrane-spanning, A, and evolutionarily conserved B domains. Each B domain binds specifically to the light chain of antibodies on B lymphocytes, creating molecular tension at the binding interface. This force stabilizes the B domains against physiological shear stress, thereby supporting bacterial immune evasion.
The research focused on Protein L, a molecule from the bacterium Finegoldia magna. Protein L is known as a “superantigen” because it can bind antibodies in unusual ways, potentially disrupting normal immune function. What makes this study remarkable is its use of single-molecule force spectroscopy — a cutting-edge technique that applies tiny, precise forces to individual molecules to see how they behave under stress.
The scientists discovered that when IgM binds to Protein L, it significantly increases the protein’s mechanical stability. In simple terms, IgM acts like a brace, making it harder for the protein to unfold or break apart under force. This effect was concentration-dependent — higher amounts of IgM gave Protein L more resistance to mechanical stress.
The team also used computer simulations to explore why this happens. They found that IgM’s multiple binding sites can engage Protein L at several points simultaneously, creating a synergistic stabilizing effect. This is different from smaller antibodies, which lack the same stabilizing power.
Many bacteria experience mechanical forces inside the human body — for example, during blood flow or immune cell attack. If IgM can neutralize toxins by making them more mechanically rigid, this opens new avenues for designing antibody-based therapies that target bacterial virulence in a completely new way.
This work highlights an underappreciated role of antibodies: not just chemical binders, but also mechanical modulators in our fight against disease.
***
NKR/PSM
(Release ID: 2160645)
Visitor Counter : 487