Ministry of Science & Technology
Morning Coffees Could Hold the Secret to Spotting Deadly Dyes in Food
प्रविष्टि तिथि:
13 OCT 2025 5:10PM by PIB Delhi
Scientists have found a simple yet effective method of detecting toxic molecules at incredibly low concentrations – by exploiting the same phenomenon that makes coffee stains.
When a coffee drop evaporates on a tabletop, it creates a distinctive dark circle around the edge. This daily phenomenon, or the coffee-stain effect, is due to the fact that as the liquid dries, particles suspended in the liquid move outward and cluster along the edge. Researchers have known for some time now that this same principle is true for droplets of other particles too, not coffee alone.
By investigating and manipulating this natural phenomenon, scientists can induce nanoparticles to align themselves into characteristic, highly ordered patterns along the edge of dry stains. These ring-shaped deposits can be used as tiny landscapes where light interacts with matter. This creates unique opportunities for detecting certain substances in amounts which would otherwise be too small to measure.
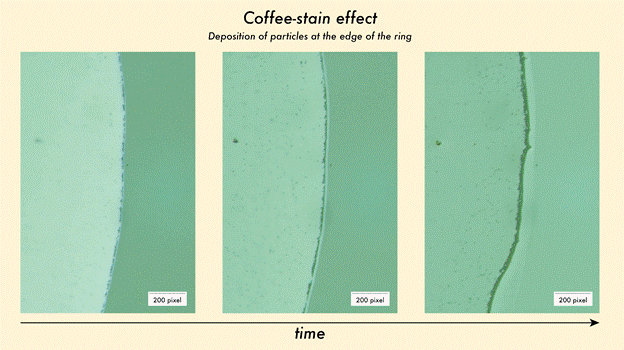
Fig 1. The movement of particles in a droplet to the edge of the ring during the drying process
Researchers at Raman Research Institute (RRI), an autonomous institute supported by the Department of Science and Technology (DST), Government of India focused on Rhodamine B, fluorescent synthetic dye used in textiles and cosmetics. However, this dye is toxic and causes damage to skin, eyes, and even the respiratory system. It is also an important environmental contaminant in that it has been reported to persist in water.
The team exploited the coffee-stain effect with gold nanorods, microscopic rods a few tens of nanometers in length by depositing a water droplet containing them on a cleaned, strongly water-attracting silicon surface and allowing the water to evaporate. As the droplet evaporated, the rods were transported to its rim and left there in a ring. Depending on how dense the rods had been in the droplet, the edge deposit could either be a thin, loosely spread-out layer or a dense, towering structure with the rods packed closely together end-to-end and side-by-side. These compact edge structures are particularly significant because they form hundreds of "hot spots" in which light is greatly intensified. When a laser is directed at the stain, any Rhodamine B molecules bound to the gold rods in these areas generate much brighter optical signals than they would individually.
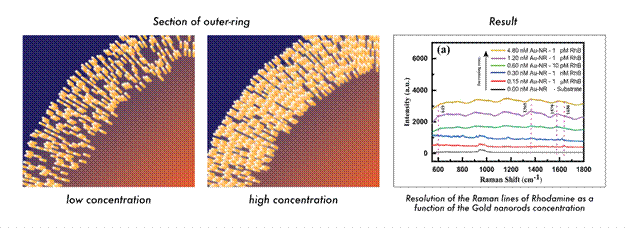
Fig 2. (Left & Middle) Artist impression of Gold Nanorods deposited at the edge of the ring at varying concentrations, (Right) Intensity profile of Rhodamine detected in increasing concentration of Gold Nanorod solution
At low concentrations of gold nanorods, only relatively high amounts of Rhodamine B could be detected – roughly equivalent to a drop of dye in a glass of water. As the nanorod concentration increased, the detection limit improved sharply. With the densest ring deposits, the system could detect Rhodamine B down to one part in a trillion. Remarkably, a hundred-fold increase in nanorod concentration translated into nearly a million-fold enhancement in sensitivity.
“Dye molecules such as Rhodamine B are banned in products such as food and cosmetics due to their toxicity, but regulators face challenges in monitoring their illegal use, including usage of small quantities in products and a lack of availability of detection equipment,” said A. W. Zaibudeen, researcher at RRI.
“Once these dyes mix with food or water bodies, they may become diluted to concentrations as low as parts per trillion making it difficult to detect them using conventional characterization techniques. Therefore, a more sensitive method of detection, such as Surface-Enhanced Raman Spectroscopy (SERS), may be required,” said Yatheendran K. M, Engineer B, Soft Condensed Matter.
Practically speaking, the scientists demonstrated that it is possible to use a simple, naturally formed pattern that is produced by the same phenomenon that produces coffee rings on a table, to transform into a fantastically potent and inexpensive technique for chemical detection.
“This technique is facile and cost-effective. After the drop of liquid evaporates, the dried pattern concentrates the nanoparticles, and the hotspots that form allow us to detect even picomolar quantities of toxins. Even hand-held Raman spectrometers could potentially be employed to detect toxins using this technique,” said Ranjini Bandyopadhyay, Professor – Soft Condensed Matter at RRI.
More generally, the technique can be used for a wide range of harmful substances, and can be transformed into advanced technology for reducing disease and environmental harm.
***
NKR/PSM
(रिलीज़ आईडी: 2178524)
आगंतुक पटल : 1687