Ministry of Science & Technology
Scientists decode new avenues for research on healthy ageing
Posted On:
13 JAN 2026 5:51PM by PIB Delhi
The “neighbourhood” around stem cells, rather than the stem cells alone, may hold a vital clue to understanding how tissues age and lose their regenerative capacity, according to new research. The study reveals that support cells surrounding stem cells are far more vulnerable to ageing-related damage, opening new avenues for research on healthy ageing.
Healthy aging is a global priority and scientists are working on strategies for delaying the onset of age-related decline in human tissues.
Researchers at the Agharkar Research Institute (ARI), Pune, an autonomous institute of the Department of Science & Technology (DST), studied the ovaries of the fruit fly Drosophila melanogaster to investigate the mechanisms that maintain the function of reproductive stem cells over time. Their study published as a cover article in the journal Stem Cell Reports, found that while germline stem cells (special adult stem cells in the testes/ovaries that continuously renew themselves and produce gamete) can cope with very low levels of autophagy, the cell’s internal “recycling” system, neighbouring support cells called cap cells, are critically dependent on this process for their long-term survival.
When autophagy-related genes, such as Atg1, Atg5, or Atg9, were selectively switched off in cap cells, these niche cells accumulated damage, lost their structure, and gradually failed to send essential maintenance signals to germline stem cells. As a result, even though the stem cells themselves remained intrinsically robust, they were eventually lost from the tissue because their supportive microenvironment collapsed.
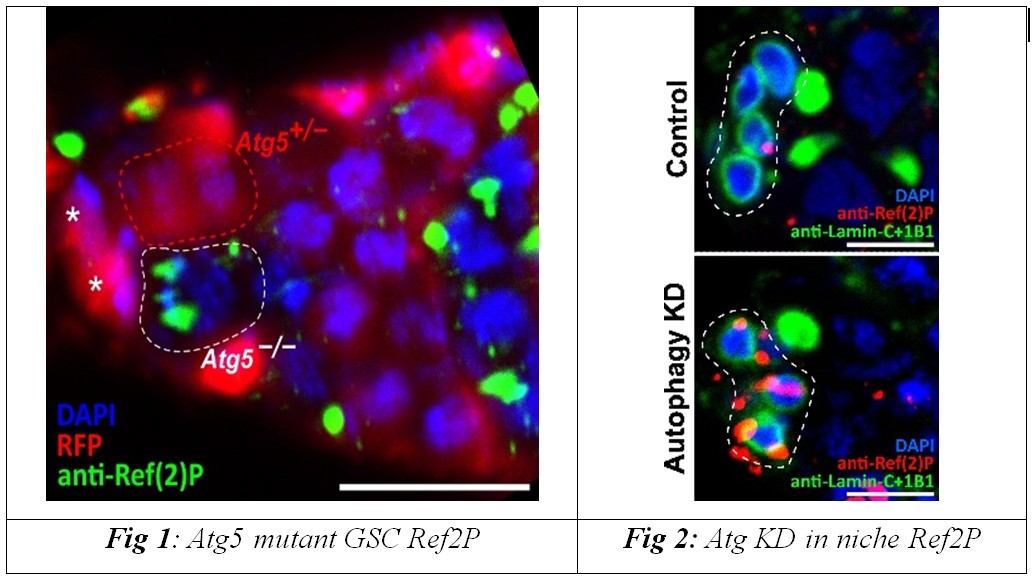
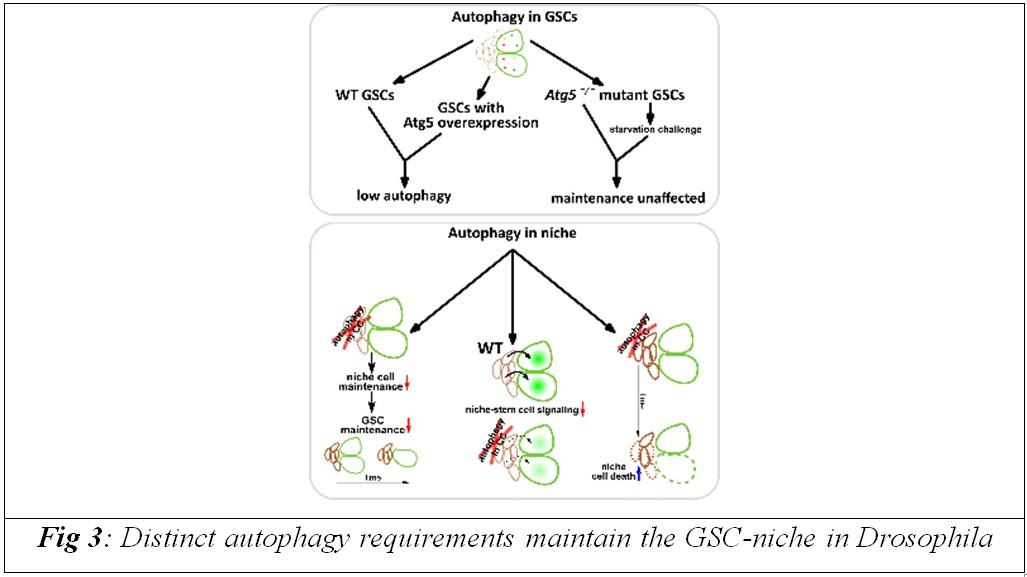
The team shows that ageing in this system does not begin with the stem cells but with the deterioration of their support cells, which act like a nurturing neighbourhood. Cap cells provide constant biochemical cues, including Bone Morphogenetic Protein (BMP) signals, that help germline stem cells maintain their identity and continue to produce eggs. When autophagy falters in these niche cells during midlife, BMP signalling weakens, and stem cells can no longer be maintained, linking microenvironmental decline directly to loss of tissue regenerative capacity.
This work challenges the traditional view that ageing is primarily driven by damage within individual cells and highlights instead a community-like process, where the fate of stem cells is closely tied to the health of neighbouring cells. By demonstrating that different cell types within the same tissue have distinct requirements for autophagy, the study highlights the importance of considering whole cellular ecosystems when designing strategies to delay aging.
The study, led by Kiran Suhas Nilangekar and Dr. Bhupendra V. Shravage at the Developmental Biology Group, Agharkar Research Institute, places ARI Pune at the forefront of research on how stem cell niches age. Their findings provide a mechanistic basis for how supportive cells can act as early “weak links” in tissues, potentially triggering age-associated decline even when stem cells themselves are relatively resilient.
By using a genetically tractable model like Drosophila, the team has generated insights that are expected to inform future work on mammalian tissues such as the intestine, skin, and muscle, where similar niche‑stem cell relationships exist.
Although the current work is conducted in fruit flies, the core pathways studied, autophagy and stem cell niche signalling, are conserved across species, making the ARI findings highly relevant to broader ageing biology. The demonstration that strengthening or protecting support cells could indirectly prolong stem cell function suggests new directions for future interventions aimed at preserving fertility and tissue health during ageing.
Going forward, researchers plan to explore how different cell types within a tissue balance resilience and fragility, and whether targeted modulation of autophagy in niche cells can slow down age-related loss of regenerative capacity.
Link to publication: https://www.cell.com/stem-cell-reports/fulltext/S2213-6711(25)00316-9
*****
NKR/FK
(Release ID: 2214197)
Visitor Counter : 835