Ministry of Science & Technology
Iron-Doped catalyst developed for sustainable oxygen electrocatalysis
Posted On:
14 MAY 2025 3:41PM by PIB Delhi
Researchers from Centre for Nano and Soft Matter Sciences, (CeNS) a Bengaluru based autonomous institute under the Department of Science and Technology (DST), have created a groundbreaking new catalyst designed to make crucial oxygen-related catalytic reactions faster, more affordable, and efficient.
Electrocatalysis involving oxygen underpins numerous clean energy technologies, such as splitting water to produce hydrogen, creating clean fuels, and manufacturing chemicals like hydrogen peroxide. However, these technologies typically face challenges like slow reaction speeds, high energy demands, and high costs due to the limited availability and expense of the precious metals involved. Traditionally, catalysts used in these processes rely on expensive precious metals like platinum or ruthenium making the processes costly.
Targeting to reduce the costs, CeNS has developed a new catalyst that uses nickel selenide enhanced by precisely adding a small amount of iron (Fe). This has the potential of not only reducing costs significantly, but also improves performance.
The team of scientists from CeNS began with a special material known as a metal-organic framework (MOF). MOFs are porous, crystalline structures useful for chemical reactions but have limited electrical conductivity. The electronic structure of the MOF has been modulated by Fe doping to improve catalytic active sites. To improve conductivity, researchers converted MOFs into carbon-rich materials through a heating process known as pyrolysis, enhancing their ability to conduct electricity effectively.
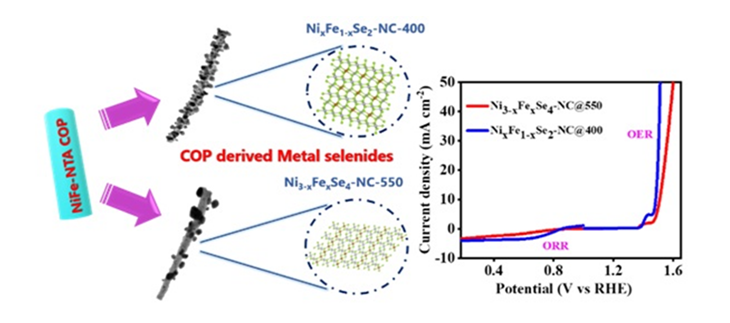
Fig: Schematic of Fe-doped Nickel selenides for bifunctional oxygen electrocatalysis
After pyrolysis, researchers introduced selenium, creating two highly effective catalysts known as NixFe1−xSe₂–NC and Ni₃−xFexSe₄–NC. The iron doping significantly improved the catalyst’s electronic interactions, creating more active sites for reactions and optimizing how reaction intermediates bind to the catalyst surface. These enhancements made the catalyst exceptionally efficient for two key processes: the Oxygen Evolution Reaction (OER), which produces oxygen, and the Oxygen Reduction Reaction (ORR), which converts oxygen into valuable chemicals.
Extensive testing by the researchers showed that the catalyst, NixFe1−xSe₂–NC@400, achieved impressive performance. For the OER process, it required significantly less energy (lower overpotential) and demonstrated superior stability over 70 hours, outperforming traditional ruthenium-based catalysts. In ORR tests for hydrogen peroxide production, this catalyst also exceeded the performance of industry-standard platinum-based catalysts, providing better reaction speeds and higher efficiency.
Additionally, the catalyst exhibited excellent electrical conductivity, a crucial feature for rapid and efficient chemical reactions. Detailed analysis revealed that iron doping changed the catalyst’s electronic structure in a beneficial way, increasing active sites and facilitating better electron transport. These changes directly enhanced the catalyst's ability to perform oxygen-related reactions, making it highly effective and durable.
This breakthrough could significantly impact industries by providing a cost-effective, sustainable, and highly efficient alternative to current catalysts. Businesses could soon benefit from catalysts that not only cut operational costs but also could reduce environmental impact.
The research published in the journal Nanoscale opens exciting new avenues for designing advanced catalysts by tuning their electronic and structural properties. This approach could lead to the widespread adoption of more affordable and sustainable catalysts in next-generation clean energy technologies.
Publication link: https://doi.org/10.1039/D4NR04047C
For further information, contact Dr. Kavita Pandey at kavitapandey[at]cens.res.in .
*****
NKR/PSM
(Release ID: 2128622)
Visitor Counter : 2